Design, synthesis and biological evaluation of novel fungicides for the management of Fusarium DieBack disease
DOI:
https://doi.org/10.29356/jmcs.v62i3.531Keywords:
Fungicide, Synthesis, Triazol, Euwallacea, FusariumAbstract
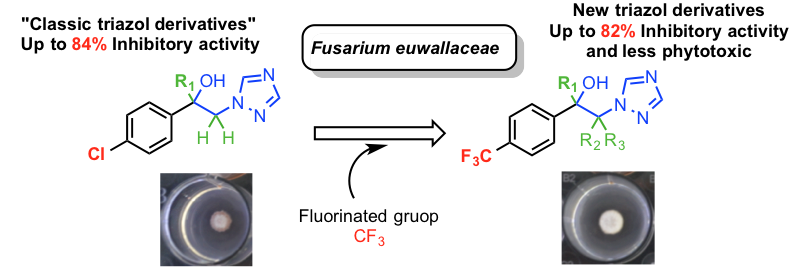
Abstract. Fusarium Dieback, a new and lethal insect-vectored disease can host over 300 tree species including the avocado trees. This problem has recently attracted the attention of synthetic chemist not only to develop new triazol antifungal agents but also due to severe drug resistance to “classic” triazol antifungal agents. Here, a series of novel antifungal triazoles with a p-trifluoromethylphenyl moiety were synthesized and characterized by spectroscopic and spectrometric methods. Most of the target compounds synthesized showed from modest to good inhibitory activity and less phytotoxicity in comparison with the commercially available propiconazol; in particular, compounds 7 and 13 were active against both Fusarium solani and Fusarium euwallaceae. The results showed that compounds 7, 13, and 4 have great potential to be developed as new antifungal agents because of both good antifungal activity and low phytotoxicity. SAR showed that free alcohols and not O-protected compounds significantly influence the activity. Hence, a-methyl-a-1,2,4-triazole emerged as novel compound to develop new ketone-triazole-type antifungal agents for the management of Fusarium Dieback disease
Resumen. Fusarium Dieback es una nueva enfermedad letal transmitida por insectos que actúan como vectores y puede atacar a más de 300 especies de árboles, incluidos los árboles de aguacate. Recientemente, este problema ha atraído la atención de los químicos sintéticos para desarrollar nuevos agentes antifúngicos triazólicos debido a la resistencia severa que desarrollan los insectos a los agentes antifúngicos triazólicos "clásicos". Durante este trabajo, se sintetizaron nuevos triazoles antifúngicos que contienen un grupo p-trifluorometilfenilo y se caracterizaron por métodos espectroscópicos y espectrométricos. La mayoría de los compuestos diana sintetizados mostraron una actividad inhibidora de modesta a buena y menos fitotoxicidad en comparación con el propiconazol que es comercialmente disponible; en particular, los compuestos 7 y 13 mostraron ser activos contra Fusarium solani y Fusarium euwallaceae. Los resultados mostraron que los compuestos 7, 13 y 4 tienen un gran potencial para desarrollarse como nuevos agentes antifúngicos debido a la buena actividad antifúngica y su baja fitotoxicidad. SAR mostró que los alcoholes libres y no los compuestos O-protegidos influyen significativamente en la actividad. Por lo tanto, el α-metil-α-1,2,4-triazol surgió como un nuevo compuesto líder para desarrollar nuevos agentes antifúngicos tipo cetona-triazol para el tratamiento de la enfermedad Fusarium Dieback.
Downloads
References
Freeman, S.; Sharon, M.; Maymon, M.; Mendel, Z.; Protasov, A.; Aoki, T.; Eskalen, A.; O’Donnell, K. Mycologia 2013, 105, 1595–1606.
Dirección General de Sanidad Vegetal, Centro Nacional de Referencia Fitosanitaria. Euwallacea sp, 2014. http://www.avocadosource.com/papers/Bulletins/DGdeSV2014b.pdf [accessed 23 January 2018]
Umeda C.; Eskalen A.; Paine T. D. Polyphagous Shot Hole Borer and Fusarium Dieback in California: Insects and Diseases of Mediterranean Forest Systems, edited by Paine, T. and Lieutier F., Springer, Cham, pp 757-767 (2016).
Batra, L. R. Proc. Indian Acad. Sci. (Plant. Sci.) 1985, 94, 137–148.
McCullough, D. G.; Mercader, R. J.; Siegert, W. Can. Entomol. 2015, 147, 349–358.
Parson, J. H.; West, P. J. U.S. Patent 4414221 1981.
Ma, Y.; Liu, R.; Gong, X.; Li, Z.; Huang, Q.; Wang, H.; Song, G. J. Agric. Food Chem. 2006, 54, 7724-7728.
a) Ferreira, E. M.; Alfenas, A. C.; Maffia, L. A.; Mafia, R.G.; Mounter, A. H. Crop Prot. 2008, 27, 161-170. b) Tang, R.; Jin, L.; Mou, C.; Yin, J.; Bai, S.; Hu, D.; Wu, J.; Yang, S.; Song, B. Chem. Cent. J. 2013, 7, 1-7.
Pfaller, M. A. Am. J. Med. 2012, 125, S3-S13.
a) Kuhle E.; Klauke, E. Angew. Chem. Int. Ed. 1977, 16, 735-742. b) Karimova, N. M.; Ignatova, J. L.; Rozhkov, I. N. J. Fluorine Chem. 1992, 58, 367-367. c) Welch, J. T. Tetrahedron 1987, 43, 3123-3197. b) The previous work developed by Minoru, T. et al. describes the synthesis of fungicides bearing the p-trifluoromethylphenyl moiety in the molecule, nonetheless, it is used against fungicidal infections on mamals. PCT Int. Appl. WO 9427976 A1 19941208 1994.
Lima, L. M. A.; Barreiro, E. J. Curr. Med. Chem 2005, 12, 23-49.
Pericherla, K.; Khedar, P.; Khungar, B.; Kumar, A. Tetrahedron Lett. 2012, 53, 6761-6764.
Monfette, S.; Turner, Z. R.; Semproni, S. P.; Chirik, P. J. J. Am. Chem. Soc. 2012, 134, 4561-4564.
a) Bogale M.; Steenkamp E. T.; Wingfield M. Eur. J. Plant Pathol. 2009, 124, 369–378.
The Chemistry of Heterocycles, Eicher, T. and Hauptmann, S. Eds. Wiley-VCH Verlag GmbH & Co. KGaA, 2003, 208-209.
Gozzo, F.; Carelli, A.; Carzaniga, R.; Farina, G.; Arnoldi, A.; Lamb, D.; Kelly, S. L. Pestic. Biochem. Physiol. 1995, 53, 10-22.
Yamazaki, T.; Taguchi, T.; Ojima, I. Unique Properties of Fluorine and their Relevance to Medicinal Chemistry and Chemical Biology: Fluorine in Medicinal Chemistry and Chemical Biology, Ojima I. Ed., John Wiley & Sons, Inc., 2009, 1-46.
Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.